- Largest To Smallest Elements
- Identify The Smallest Unit Of An Element
- The Smallest Unit Of An Element Is Called
The smallest unit of an element that still retains the distinctive behavior of that element is an. Atom The characteristic that gives an element its distinctive properties is its number of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Learning Objective
- Discuss the electronic and structural properties of an atom
Key Points
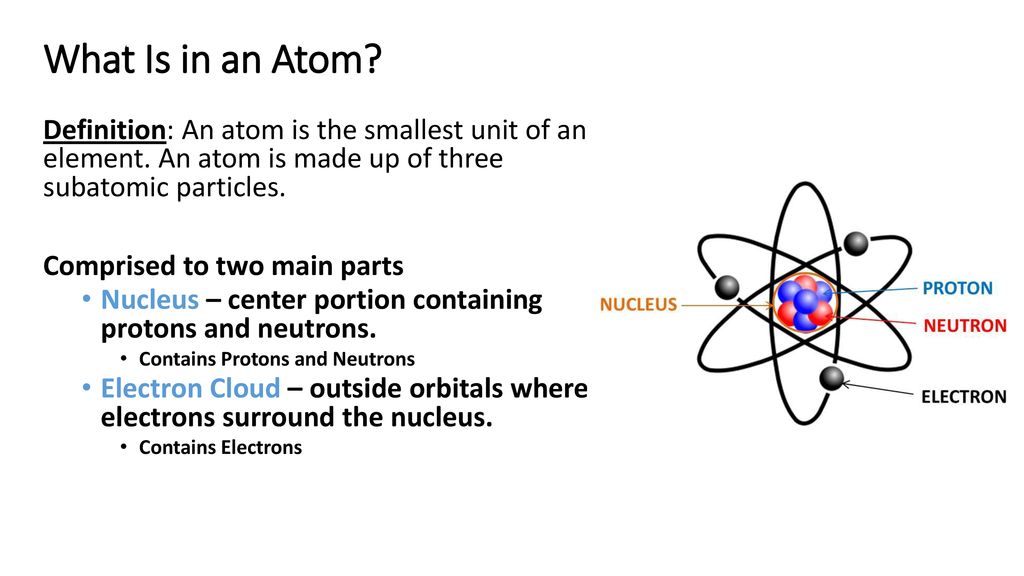
- An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.
- Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which scientists define as one atomic mass unit (amu) or one Dalton.
- Each electron has a negative charge (-1) equal to the positive charge of a proton (+1).
- Neutrons are uncharged particles found within the nucleus.
Terms
- protonPositively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an element. It weighs 1 amu.
- atomThe smallest possible amount of matter which still retains its identity as a chemical element, consisting of a nucleus surrounded by electrons.
- neutronA subatomic particle forming part of the nucleus of an atom. It has no charge. It is equal in mass to a proton or it weighs 1 amu.
An atom is the smallest unit of matter that retains all of the chemical properties of an element. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. Many biological processes are devoted to breaking down molecules into their component atoms so they can be reassembled into a more useful molecule.
Atomic Particles
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles.
The hydrogen atom (H) contains only one proton, one electron, and no neutrons. This can be determined using the atomic number and the mass number of the element (see the concept on atomic numbers and mass numbers).
Atomic Mass
Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams. Scientists define this amount of mass as one atomic mass unit (amu) or one Dalton. Although similar in mass, protons are positively charged, while neutrons have no charge. Therefore, the number of neutrons in an atom contributes significantly to its mass, but not to its charge.
Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. Therefore, they do not contribute much to an element’s overall atomic mass. When considering atomic mass, it is customary to ignore the mass of any electrons and calculate the atom’s mass based on the number of protons and neutrons alone.
Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Scientists define these charges as “+1” and “-1. ” In an uncharged, neutral atom, the number of electrons orbiting the nucleus is equal to the number of protons inside the nucleus. In these atoms, the positive and negative charges cancel each other out, leading to an atom with no net charge.
Volume of Atoms
Accounting for the sizes of protons, neutrons, and electrons, most of the volume of an atom—greater than 99 percent—is, in fact, empty space. Despite all this empty space, solid objects do not just pass through one another. The electrons that surround all atoms are negatively charged and cause atoms to repel one another, preventing atoms from occupying the same space. These intermolecular forces prevent you from falling through an object like your chair.
 Show Sources
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/neutron
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/electron
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/proton
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/atom
Wiktionary
CC BY-SA 3.0.
http://cnx.org/content/m14044/latest/
OpenStax CNX
CC BY 3.0.
http://cnx.org/content/m44390/latest/?collection=col11448/latest
OpenStax CNX
CC BY 3.0.
http://cnx.org/content/m44390/latest/Figure_02_01_01.jpg
OpenStax CNX
CC BY 3.0.
c. an atom
Explanation:
C
Explanation:
1. C
2. A
3. C
Action replay dsi firmware update 1.71. Bishop idahosa preaching. 4. B
5. D
Explanation:
i answered it all for you.

when [oh-] is 10-5 m, and [h3o+] is 10-9 m, the poh is 5 and ph is 9. again in both cases the sum of ph and poh is 14. however, the hydrogen ion concentration is not always going to be equal to exactly 1 x 10 raised to a negative number. for example, we skipped over the value of 2.0 x 10-7.

Largest To Smallest Elements
Identify The Smallest Unit Of An Element
The Smallest Unit Of An Element Is Called
